Unknown Facts About Amino Acid Raw Materials Manufacturers Made Known
페이지 정보

본문
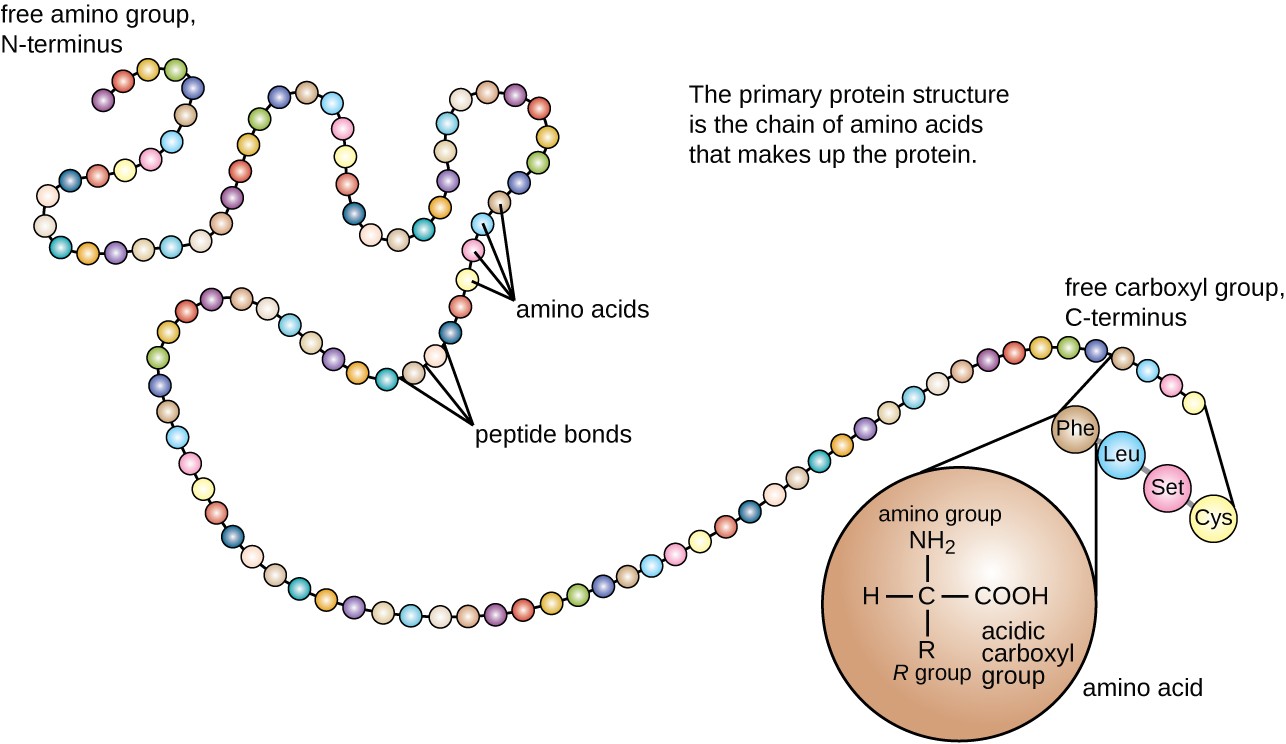 Objective The key goal of an inspection is to find out whether the producer is operating in a state of management and in compliance with the laws and laws. Although the level of know-how is increasing, it have to be recognized that the same fundamental laws and necessities are applicable to the manufacture and control of biotechnically- derived and devices as for "conventionally" manufactured merchandise. The feed additives market research report offers an in depth evaluation of the market and focuses on key points resembling main companies, animal sorts, and leading varieties of products. Many companies are forming mergers, acquisitions, partnerships, and deploying collaborative strategies to enable market growth. Included listed here are additionally particular amino acids, radioactively labeled and unnatural products in addition to in another way configured compounds. Special due to Ms. Kimberly Search (DFI) for her skilled clerical help. This Guide was initiated by Robert C. Fish, Director, Division of Field Investigations (DFI). Revenues from the newly acquired entity could be included in Evonik’s sales and earnings for the yr 2020. The corporate, with its staff and the corresponding production facilities, built-in into the Smart Materials division of Evonik. However, worth and provide fluctuations of raw materials are expected to slow down the expansion of the market.
Objective The key goal of an inspection is to find out whether the producer is operating in a state of management and in compliance with the laws and laws. Although the level of know-how is increasing, it have to be recognized that the same fundamental laws and necessities are applicable to the manufacture and control of biotechnically- derived and devices as for "conventionally" manufactured merchandise. The feed additives market research report offers an in depth evaluation of the market and focuses on key points resembling main companies, animal sorts, and leading varieties of products. Many companies are forming mergers, acquisitions, partnerships, and deploying collaborative strategies to enable market growth. Included listed here are additionally particular amino acids, radioactively labeled and unnatural products in addition to in another way configured compounds. Special due to Ms. Kimberly Search (DFI) for her skilled clerical help. This Guide was initiated by Robert C. Fish, Director, Division of Field Investigations (DFI). Revenues from the newly acquired entity could be included in Evonik’s sales and earnings for the yr 2020. The corporate, with its staff and the corresponding production facilities, built-in into the Smart Materials division of Evonik. However, worth and provide fluctuations of raw materials are expected to slow down the expansion of the market.
Lack of customary steerage for fingerprinting cell tradition media and related raw materials. A cell seed lot consists of aliquots of a single culture. CLONE - A cell line stemming from a single ancestral cell and usually expressing all the identical genes. The cell seed lot system is used by manufacturers to guarantee identification and purity of the starting raw materials. Secondly, the purity of amino acids is crucial, and impurities may cause problems in downstream processes. This Guide will tackle some of the essential issues recognized throughout inspections of manufacturers of BDP. Otherwise the dishes might be tasteless and will not profit the physique. Even if the physique is able to producing these substances on its own, non-important amino acids can also be obtained from food. Analysts (Chemists and/or Microbiologists), Computer Specialists, and Engineers can take part in all or elements of the inspection. One vital side of an inspection is to identify defective product, non-conforming product and system failures.
 It must be emphasised that the assessments required to characterize a cell bank will rely on the intended use of the final product, the host/expression system and the tactic of manufacturing including the methods employed for purification of the product. They are also essential substances in pet meals merchandise and veterinary supplements to enhance food digestibility and strengthen the immune system. Feed additives and ingredients play an vital role in alleviating monetary pressures and uncertainties, ensuring that animals obtain satisfactory nutritional meals. By reducing the quantity of waste produced by animals and the carbon footprint of feed manufacturing, the amino acid-primarily based feed can even help lessen the environmental impression of livestock farming. Likewise, farmers and breeders use a type of "genetic engineering" to supply improved crops and inventory by selecting for fascinating characteristics in plants and animals. As well as, their use in nutritional sports supplements is turning into more and more in style. In addition, the forms of exams might change as expertise advances. Generally, cells saved in liquid nitrogen or its vapor section are stable longer than cells saved at -70 C. In addition, it is recommended that the MCB and WCB be saved in a couple of location within the event that a freezer malfunctions.
It must be emphasised that the assessments required to characterize a cell bank will rely on the intended use of the final product, the host/expression system and the tactic of manufacturing including the methods employed for purification of the product. They are also essential substances in pet meals merchandise and veterinary supplements to enhance food digestibility and strengthen the immune system. Feed additives and ingredients play an vital role in alleviating monetary pressures and uncertainties, ensuring that animals obtain satisfactory nutritional meals. By reducing the quantity of waste produced by animals and the carbon footprint of feed manufacturing, the amino acid-primarily based feed can even help lessen the environmental impression of livestock farming. Likewise, farmers and breeders use a type of "genetic engineering" to supply improved crops and inventory by selecting for fascinating characteristics in plants and animals. As well as, their use in nutritional sports supplements is turning into more and more in style. In addition, the forms of exams might change as expertise advances. Generally, cells saved in liquid nitrogen or its vapor section are stable longer than cells saved at -70 C. In addition, it is recommended that the MCB and WCB be saved in a couple of location within the event that a freezer malfunctions.
The WCB is defined as a amount of cells derived from one or more ampules of the MCB, saved cryogenically and used to provoke the manufacturing batch. These methods are extra exact, however the outcomes are just like those produced with classical genetic strategies involving whole organisms. Only just lately have "new" biotechnology methods enabled scientists to change an organism's genetic material on the cellular or molecular degree. Biotechnology - derived merchandise (BDP) used on this Guide refers to these products derived from the brand new biotechnology strategies. Mr. Fish requested Barbara-Helene mith, Ph.D., DIB, CHI-DO, to chair a workgroup to develop inspectional guidelines for Investigators in the world of biotechnology. Note: This doc is reference materials for investigators and different FDA personnel. The workgroup, which also included Thaddeus T. Sze, Ph.D., Chemical Engineer, DFI, and Kim A. Rice, Supervisory Investigator, SEA-DO, ready a draft document with info obtained from FDA Center and Field personnel who're actively concerned in biotech inspections.
- 이전글The Highstakes Online Trap 25.02.06
- 다음글The Most Effective Reasons For People To Succeed On The Compact Single Stroller Industry 25.02.06
댓글목록
등록된 댓글이 없습니다.



